Abstract: The RInChI is a canonical identifier for reactions which is widely used in reaction databases. It can be used to handle large collections of reactions and to link information from diverse data sources. How much information can it handle? Studies of the SAVI database, which contains more than a […]
InChI Tag: Miscellaneous
Evaluated Kinetic Data for Atmospheric Chemistry Description The IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation is continuing to update and expand the evaluations, which have been published in a series of ten peer reviewed articles in J. Phys. Chem. Ref. Data, and six recent articles in Atmos. Chem. Phys. journals. […]
A database of chemical structures and identifiers used in the control of WADA Prohibited Substances Description WADA’s role is to help regulate doping internationally working with international sports federations and governments. The list of regulated substances is published annually (https://www.wada-ama.org/en/what-we-do/the-prohibited-list), with substances listed in sub-categories (e.g. stimulants and anabolic agents) […]
WorldFAIR Chemistry – 2. Training Cookbook: Digital recipes for managing chemical data Description This project corresponds to Deliverable 3.2 of the WorldFAIR Chemistry project. The main scope of work will be to develop actionable recipes in the format of a digital Cookbook for how to prepare and deposit FAIR machine-enabled […]
InChIs and Registry Numbers by Jeffery Leigh From the journal Chemistry International — Newsmagazine for IUPAC https://doi.org/10.1515/ci.2012.34.6.23
PubChem: Advancing chemical information through InChI Evan Bolton InChI in the Wild: Celebrating Over 20 years of InChI Development in Memory of InChI Developer Igor PletnevFall 2022 ACS National Meeting2022-08-21 Abstract: The PubChem project (https://pubchem.ncbi.nlm.nih.gov) has been a long time user and contributor to the InChI project. The impact of the InChI project on […]
InChI QR code generator https://www-rinchi.ch.cam.ac.uk/qrinchi/ J.M. Goodman & E.D.J. Goodman This is an experimental, proof-of-concept, QRInChI generator. IUPAC is discussing a standard for InChI QR Codes, but a recommendation has yet to be agreed. These QRInChI are generated without any guarantee for any application whatsoever.
Tautomerism in large databases Markus Sitzmann, Wolf-Dietrich Ihlenfeldt, and Marc C. Nicklaus https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2886898/ Journal of Computer-Aided Molecular Design volume 24, pages 521-551 (2010) https://link.springer.com/article/10.1007/s10822-010-9346-4 Abstract: We have used the Chemical Structure DataBase (CSDB) of the NCI CADD Group, an aggregated collection of over 150 small-molecule databases totaling 103.5 million […]
Tautomer Database: A Comprehensive Resource for Tautomerism Analyses Devendra K. Dhaked, Laura Guasch, and Marc C. Nicklaus https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8456363/ Abstract: We report a database of tautomeric structures that contains 2819 tautomeric tuples extracted from 171 publications. Each tautomeric entry has been annotated with experimental conditions reported in the respective publication, […]
ChemNames2LCSS This Google spreadsheet will allow anyone to connect a list of up to 1,000 chemical names to safety chemical information available in PubChem LCSS. To obtain the spreadsheet you click the content link, which directs you to Google Docs and allows you to make your own copy of the […]
The RInChI Project The aim of the RInChI Project is to create a unique, canonical, text string to describe a reaction. Different researchers working on the same reaction should be able to generate the same RInChI without needing to confer with each other https://www-rinchi.ch.cam.ac.uk/ International chemical identifier for chemical reactions 8th German […]
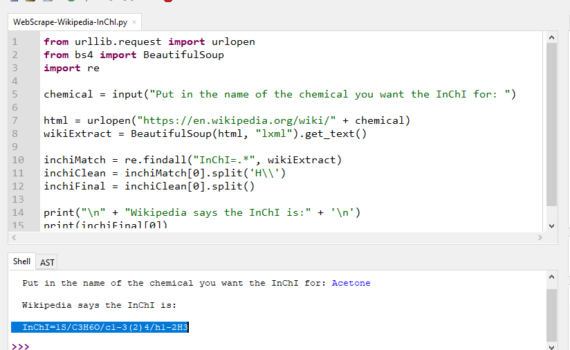
Andrew P. Cornell, Robert E. Belford Chemistry Department, University of Arkansas at Little Rock, Little Rock, Arkansas 72204 Abstract Many individual chemicals have a specific page on Wikipedia that will give information about the use, manufacture and properties of that chemical. The properties that are displayed off to the […]
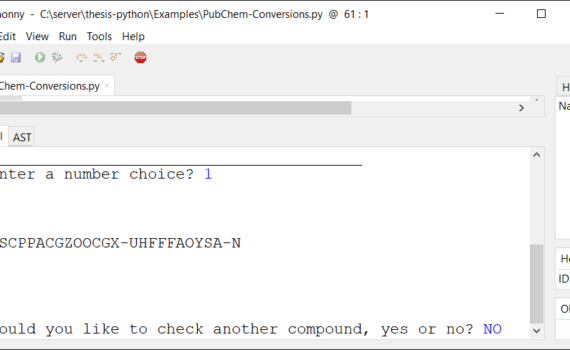
Andrew P. Cornell, Robert E. Belford Chemistry Department, University of Arkansas at Little Rock, Little Rock, Arkansas 72204 Abstract In this tutorial, a program written in Python will take a user specified chemical name and retrieve the associated chemical identifier or basic property using an online chemical database. This […]
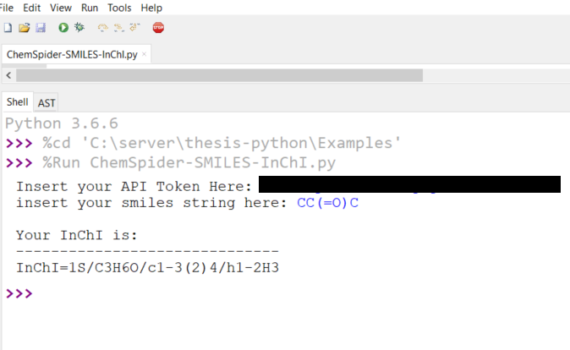
Andrew P. Cornell, Robert E. Belford Chemistry Department, University of Arkansas at Little Rock, Little Rock, Arkansas 72204 Abstract ChemSpider offers many methods in which to access online data through web API (Application Programming Interface) interactions.1 This tutorial will explain how to write a few simple lines of code […]
PubChem chemical structure standardization Volker D. Hähnke, Sunghwan Kim & Evan E. Bolton Journal of Cheminformatics volume 10, Article number: 36 (2018) Background: PubChem is a chemical information repository, consisting of three primary databases: Substance, Compound, and BioAssay. When individual data contributors submit chemical substance descriptions to Substance, the unique […]
Chemical Entity Semantic Specification: Knowledge representation for efficient semantic cheminformatics and facile data integration Leonid L Chepelev & Michel Dumontier Journal of Cheminformatics volume 3, Article number: 20 (2011 Abstract: Background: Over the past several centuries, chemistry has permeated virtually every facet of human lifestyle, enriching fields as diverse as medicine, agriculture, manufacturing, warfare, and electronics, among […]
Open Data, Open Source and Open Standards in chemistry: The Blue Obelisk five years on Journal of Cheminformatics volume 3, Article number: 37 (2011) Noel M O’Boyle, Rajarshi Guha, Egon L Willighagen, Samuel E Adams, Jonathan Alvarsson, Jean-Claude Bradley, Igor V Filippov, Robert M Hanson, Marcus D Hanwell, Geoffrey R […]
UniChem: a unified chemical structure cross-referencing and identifier tracking system Jon Chambers, Mark Davies, Anna Gaulton, Anne Hersey, Sameer Velankar, Robert Petryszak, Janna Hastings, Louisa Bellis, Shaun McGlinchey & John P Overington Journal of Cheminformatics volume 5, Article number: 3 (2013) Abstract: UniChem is a freely available compound identifier mapping […]
Consistency of systematic chemical identifiers within and between small-molecule databases Saber A Akhondi, Jan A Kors & Sorel Muresan Journal of Cheminformatics volume 4, Article number: 35 (2012) Abstract Background: Correctness of structures and associated metadata within public and commercial chemical databases greatly impacts drug discovery research activities such as […]
Detection of IUPAC and IUPAC-like chemical names Roman Klinger, Corinna Kolá?ik, Juliane Fluck, Martin Hofmann-Apitius, Christoph M. Friedrich Bioinformatics, Volume 24, Issue 13, July 2008, Pages i268–i276, https://doi.org/10.1093/bioinformatics/btn181 Motivation: Chemical compounds like small signal molecules or other biological active chemical substances are an important entity class in life science publications and patents. Several representations and nomenclatures […]
How Many Miles Have We Gone, InChI by InChI? by Alex Tropsha and Antony Williams Chemistry International — Newsmagazine for IUPAC https://doi.org/10.1515/ci.2012.34.5.33
InChIKey collision resistance: an experimental testing Igor Pletnev, Andrey Erin, Alan McNaught, Kirill Blinov, Dmitrii Tchekhovskoi & Steve Heller Journal of Cheminformatics volume 4, Article number: 39 (2012) InChIKey is a 27-character compacted (hashed) version of InChI which is intended for Internet and database searching/indexing and is based on an […]
InChI: connecting and navigating chemistry Antony J Williams Journal of Cheminformatics volume 4, Article number: 33 (2012) Abstracrt: The International Chemical Identifier (InChI) has had a dramatic impact on providing a means by which to deduplicate, validate and link together chemical compounds and related information across databases. Its influence has […]
MMsDusty: an Alternative InChI-Based Tool to Minimize Chemical Redundancy Molecular Informatics, Volume32, Issue8, August 2013, Pages 681-684 Marco Fanton, Matteo Floris, Andrea Cristiani, Stefania Olla, Ricardo Medda, Davide Sabbadin, Alessandro Bulfone, Stefano Moro MMsDusty is an alternative web-oriented InChI-based normalization tool developed with the specific aim to efficiently analyze and remove chemical redundancy, […]
InChI in the wild: an assessment of InChIKey searching in Google Christopher Southan Journal of Cheminformatics 2013, 5:10 http://www.jcheminf.com/content/5/1/10 Abstract: While chemical databases can be queried using the InChI string and InChIKey (IK) the latter was designed for open-web searching. It is becoming increasingly effective for this since more sources […]
On InChI and evaluating the quality of cross-reference links Jakub Galgonek & Ji?í Vondrášek Journal of Cheminformatics volume 6, Article number: 15 (2014) Background: There are many databases of small molecules focused on different aspects of research and its applications. Some tasks may require integration of information from various databases. […]
Webinar: Solving the issues in standardisation of stereochemical representations from Chemistry World on Vimeo. Sponsored by Bio-Rad Laboratories Abstract: Learn about technology that solves the issue of interpreting 3D stereochemical information implied in 2D structure representations.
The Semantic Chemical Web: GoogleInChI and other Mashups Peter Murray-Rust at Google Tech Talks Sept 13, 2006
This RDKit InChI Calculation with Jupyter Notebook tutorial is useful to teach the basics of how to interact with InChI using a cheminformatics toolkit in a Jupyter Notebook. The notebook has the following learning objectives: Setup RDKit with a Jupyter Notebook Construct a molecule (RDKit molecular object) from a SMILES […]
Common tools for conversions, including some spreadsheet-based options included in this site, are hard to use for hundred or thousands of compounds we may want to use in cheminformatics projects. This resource includes a diferent approach to the conversion. By using the PubChem Power User Gateway it allows converting hundreds […]
Registration system of mcule: InChI is the key 2012 San Diego ACS presentation Mcule provides virtual screening services on the web to help identifying novel drug candidates by screening different databases. For these databases, it is essential to have a robust molecule registration system not depending on different drawing […]
QSAR-modeling of toxicity of organometallic compounds by means of the balance of correlations for InChI-based optimal descriptors Toropov, A. A., Toropova, A. P., & Benfenati, E. (2010 Molecular diversity, 14(1), 183-192. This paper present a use of InChI-based molecular descriptors to predict toxicity. Its abstract follows. “Quantitative structure“activity relationships (QSAR) […]
The Chemical Translation Service—a web-based tool to improve standardization of metabolomic reports Gert Wohlgemuth, Pradeep Kumar Haldiya, Egon Willighagen, Tobias Kind, Oliver Fiehn Author Notes Bioinformatics, Volume 26, Issue 20, October 2010, Pages 2647–2648, https://doi.org/10.1093/bioinformatics/btq476 Summary: Metabolomic publications and databases use different database identifiers or even trivial names which disable queries […]
Chemistry International, Volume 38, Issue 3-4, Pages 24–26 Abstract Progress in science has always been driven by data as a primary research output. This is especially true of the data-centric fields of molecular sciences. Scholarly journals in chemistry in the 19th century captured a (probably small) proportion of research data […]
Spjuth et al. Journal of Cheminformatics 2013, 5:14 Abstract Background: The InChI algorithms are written in C++ and not available as Java library. Integration into software written in Java therefore requires a bridge between C and Java libraries, provided by the Java Native Interface (JNI) technology. Results: We here describe […]
CVDHD: a cardiovascular disease herbal database for drug discovery and network pharmacology Jiangyong Gu, Yuanshen Gui, Lirong Chen, Gu Yuan & Xiaojie Xu Journal of Cheminformatics volume 5, Article number: 51 (2013) Abstract Background: Cardiovascular disease (CVD) is the leading cause of death and associates with multiple risk factors. Herb […]
This is a collection of Matlab scripts for working with InChIKeys:Â IKextract, IKfreqFH, IKstring, and IKmusic IKextract, InChIKey Extract, can extract InChIKeys from chemical Structure data files (SDFs). This script was successfully used to extract over 90 million InChIKeys (unique chemical identifiers) from over 5000 PubChem SD files. Users can also […]
Comprehensive 2015 article published in Springer’s Journal of Computer-Aided Molecular Design. Here is the abstract, The IUPAC International Chemical Identifier (InChI) is a non-proprietary, international standard to represent chemical structures. It was conceived 15 years ago, and has been is use for 10 years. The InChI Trust is developing and […]
This is an article in Catalan that provides an introduction to chemical information and describes InChI along with other chemical identifiers. Its abstract reads: “Chemical information, once managed in books paradigmatically in Chemical Abstracts and several handbooks, has now migrated to Internet. Nowadays many large databases, both commercial and freely […]