The following worksheet and key is designed for organic chemistry students and can download and modified as needed. Learning Objectives: Isotopomers and isotopologues are analogous molecules that differ by isotope composition and/or position within a molecule. Isotopomers have known isotopic position, while isotopologues have unknown isotopic position with a molecule. […]
InChI Tag: Cheminformatics
Online mixtures demo, with MInChI generator Cheminformatics 2.0 Blog Post, Alex M. Clark The above blog goes over how to use the MInChI Demo MInChI Demo
Abstract: The RInChI is a canonical identifier for reactions which is widely used in reaction databases. It can be used to handle large collections of reactions and to link information from diverse data sources. How much information can it handle? Studies of the SAVI database, which contains more than a […]
WorldFAIR Chemistry – 2. Training Cookbook: Digital recipes for managing chemical data Description This project corresponds to Deliverable 3.2 of the WorldFAIR Chemistry project. The main scope of work will be to develop actionable recipes in the format of a digital Cookbook for how to prepare and deposit FAIR machine-enabled […]
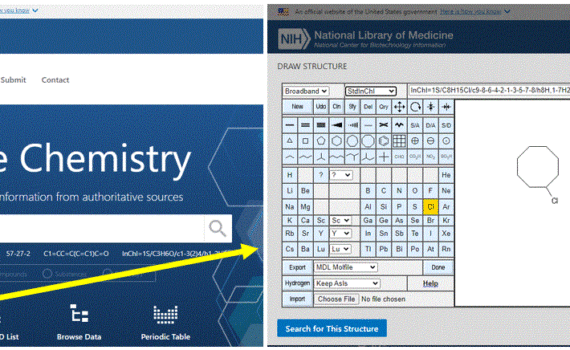
The PubChem chemical structure sketcher Sketcher Direct Link Wolf D Ihlenfeldt, Evan E Bolton & Stephen H Bryant Journal of Cheminformatics volume 1, Article number: 20 (2009) Abstract: PubChem is an important public, Web-based information source for chemical and bioactivity information. In order to provide convenient structure search methods on […]
CheMagic Virtual Molecular Model Kit The CheMagic Virtual Molecular Model Kit was developed by Otis Rothenberger and Jim Web. This molecular editor uses semantic identifiers to connect to a variety on online resources and services. If you click the GetIDdata tab for any chemical in the editor it will provide […]
MolView https://molview.org/ is a web app developed by Herman Bergwerf that uses semantic identifiers like the InChI to connect to chemical information in the cloud. If you go to any molecule and access the “information card” under tools, you will see the InChI and InChI key for that molecule. The […]
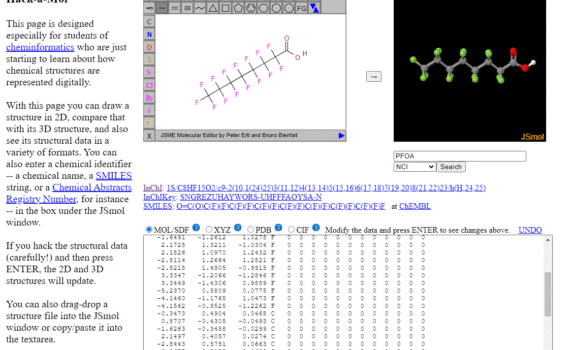
Hack-a-Mol Hack-a-Mol is a web app Bob Hanson constructed for students interested in cheminformatics. It is an interface that allows you enter names that are searched in PubChem or the NCI Resolver, and then it provides the InChI, InChI Key, SMILES and a connection table of that compound, along with […]
Progress towards “Large Molecule” support with InChI Evan Bolton,Ph.D. NCI NIH Virtual Workshop on InChI 2021-03-22 Direct Link (Alows you to activate “Full Screen mode” and open as a single slide show)
PubChem: Advancing chemical information through InChI Evan Bolton InChI in the Wild: Celebrating Over 20 years of InChI Development in Memory of InChI Developer Igor PletnevFall 2022 ACS National Meeting2022-08-21 Abstract: The PubChem project (https://pubchem.ncbi.nlm.nih.gov) has been a long time user and contributor to the InChI project. The impact of the InChI project on […]
InChI for Inorganics Alex M. Clark Cheminformatics 2.0 blog September 10, 2019 This blog post goes over extending InChI to inorganic compounds and goes over a variety of bonding patterns that could be of interest to educators. There is also a link to the GitHub that has a lot of […]
Coordination InChI for inorganics: now with stereochemistry Cheminformatics 2.0 blog by Alex Clark October 18, 2020 Cheminformatics 2.0 Blog Post GitHub: Coordination Complexes for InChI: preliminary study GitHub: Coordination Complexes for InChI: phase 2 study
Mixtures InChI: A story of how stadards drive upstream products Alex M. Clark & Leah R. McEwen NCI Virtual Workshop on international Chemical Identifiers, March 22, 2021 Video Presentation Presentation
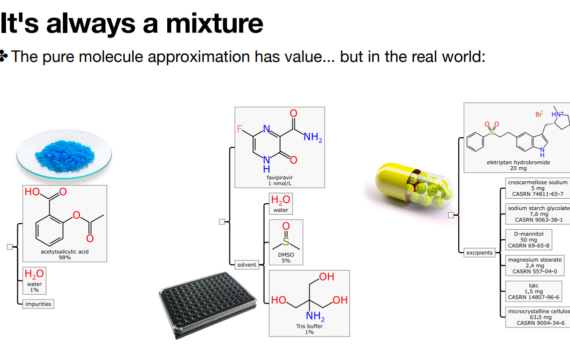
Mixtures as first class citizens in the realms of informatics Alex M. Clark, Collaborative Drug Discovery Cambridge Cheminformatics Network Meeting February 10, 2021 YouTube recording (starts at 1:06) Slideshare Slides
Mixtures: Informatics for formulations and consumer products Leah R. McEwen & Alex M. Clark Presentation to the Royal Society of Chemistry Formulation 4.1 https://www.formulation.org.uk/f4p1programme/253-past/2020/form4p1/783-form4p1-clark.html (Video) Slides: https://www.slideshare.net/aclarkxyz/mixtures-informatics-for-formulations-and-consumer-products
Tautomers in InChI NIH InChI Workshop, March 22, 2021 ? https://cactus.nci.nih.gov/presentations/NIHInChI_2021-03/Day_1_Nicklaus_Tautomerism_2021-03-21A.pdf Presentation by Marc C. Nicklaus Redesign of Handling of Tautomerism for InChI V2 IUPAC Project 2012-023-2-800
Tautomerizer – Predict tautomers based on 80+ rules https://cactus.nci.nih.gov/tautomerizer/ Introduction from Web Service (11/24/2022): Experimental service that allows you to test a set of tautomeric transforms with your own molecules. The predefined set of transforms comprises both the current 24 standard rules used by the chemoinformatics toolkit CACTVS and 55+ additional […]
Enumeration of Ring – Chain Tautomers Based on SMIRKS Rules Laura Guasch, Markus Sitzmann, and Marc C. Nicklaus https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4170818/ J Chem Inf Model. 2014 Sep 22; 54(9): 2423 – 2432. Abstract: A compound exhibits (prototropic) tautomerism if it can be represented by two or more structures that are related by […]
Tautomerism of Warfarin: Combined Chemoinformatics, Quantum Chemical, and NMR Investigation Laura Guasch, Megan L. Peach, and Marc C. Nicklaus https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7724503/ J Org Chem. 2015 Oct 16; 80(20): 9900-9909. Abstract: Warfarin, an important anticoagulant drug, can exist in solution in 40 distinct tautomeric forms through both prototropic tautomerism and ring chain […]
Experimental and Chemoinformatics Study of Tautomerism in a Database of Commercially Available Screening Samples Laura Guasch, Waruna Yapamudiyansel, Megan L. Peach, James A. Kelley, Joseph J. Barchi, Jr., and Marc C. Nicklaus https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5129033/ J Chem Inf Model. 2016 Nov 28; 56(11): 2149 – 2161. Abstract: We investigated how many cases […]
Tautomerism in large databases Markus Sitzmann, Wolf-Dietrich Ihlenfeldt, and Marc C. Nicklaus https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2886898/ Journal of Computer-Aided Molecular Design volume 24, pages 521-551 (2010) https://link.springer.com/article/10.1007/s10822-010-9346-4 Abstract: We have used the Chemical Structure DataBase (CSDB) of the NCI CADD Group, an aggregated collection of over 150 small-molecule databases totaling 103.5 million […]
Crowdsourced Evaluation of InChI-based Tautomer Identification precisionFDA Challenge https://precision.fda.gov/challenges/29 Challenge Time Period November 1, 2022 – March 1, 2023 This challenge focuses on the International Chemical Identifier (InChI), which was developed and is maintained under the auspices of the International Union of Pure and Applied Chemistry (IUPAC) and the InChI Trust. […]
Why is Isotopic Labeling Useful in Metabolomics? 2019-06-24 Metabolomics Workshop Introduction Slide Show
Figshare repository of the InChI Isotopologue and Isotopomer Development Team lead by Hunter Moseley. Provides some definitions that could be of value to educators who wish to expound upon the nuances of isotopologues and isotopomers.
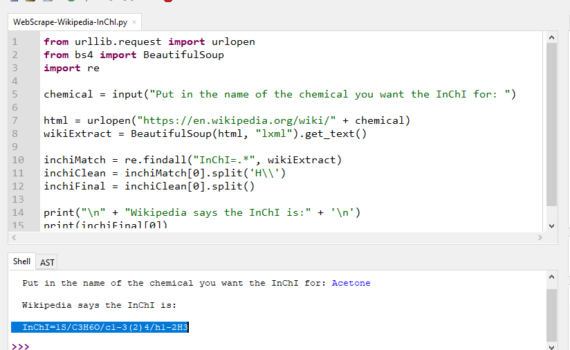
Andrew P. Cornell, Robert E. Belford Chemistry Department, University of Arkansas at Little Rock, Little Rock, Arkansas 72204 Abstract Many individual chemicals have a specific page on Wikipedia that will give information about the use, manufacture and properties of that chemical. The properties that are displayed off to the […]
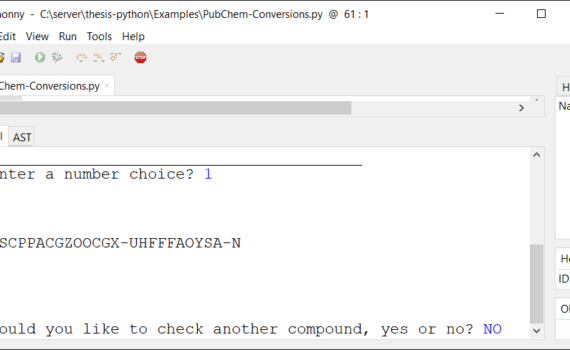
Andrew P. Cornell, Robert E. Belford Chemistry Department, University of Arkansas at Little Rock, Little Rock, Arkansas 72204 Abstract In this tutorial, a program written in Python will take a user specified chemical name and retrieve the associated chemical identifier or basic property using an online chemical database. This […]
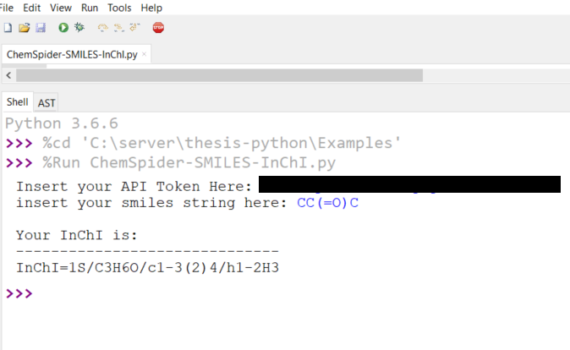
Andrew P. Cornell, Robert E. Belford Chemistry Department, University of Arkansas at Little Rock, Little Rock, Arkansas 72204 Abstract ChemSpider offers many methods in which to access online data through web API (Application Programming Interface) interactions.1 This tutorial will explain how to write a few simple lines of code […]
PubChem chemical structure standardization Volker D. Hähnke, Sunghwan Kim & Evan E. Bolton Journal of Cheminformatics volume 10, Article number: 36 (2018) Background: PubChem is a chemical information repository, consisting of three primary databases: Substance, Compound, and BioAssay. When individual data contributors submit chemical substance descriptions to Substance, the unique […]
Chemical Entity Semantic Specification: Knowledge representation for efficient semantic cheminformatics and facile data integration Leonid L Chepelev & Michel Dumontier Journal of Cheminformatics volume 3, Article number: 20 (2011 Abstract: Background: Over the past several centuries, chemistry has permeated virtually every facet of human lifestyle, enriching fields as diverse as medicine, agriculture, manufacturing, warfare, and electronics, among […]
Open Data, Open Source and Open Standards in chemistry: The Blue Obelisk five years on Journal of Cheminformatics volume 3, Article number: 37 (2011) Noel M O’Boyle, Rajarshi Guha, Egon L Willighagen, Samuel E Adams, Jonathan Alvarsson, Jean-Claude Bradley, Igor V Filippov, Robert M Hanson, Marcus D Hanwell, Geoffrey R […]
UniChem: a unified chemical structure cross-referencing and identifier tracking system Jon Chambers, Mark Davies, Anna Gaulton, Anne Hersey, Sameer Velankar, Robert Petryszak, Janna Hastings, Louisa Bellis, Shaun McGlinchey & John P Overington Journal of Cheminformatics volume 5, Article number: 3 (2013) Abstract: UniChem is a freely available compound identifier mapping […]
Consistency of systematic chemical identifiers within and between small-molecule databases Saber A Akhondi, Jan A Kors & Sorel Muresan Journal of Cheminformatics volume 4, Article number: 35 (2012) Abstract Background: Correctness of structures and associated metadata within public and commercial chemical databases greatly impacts drug discovery research activities such as […]
Towards a Universal SMILES representation – A standard method to generate canonical SMILES based on the InChI Noel M O’Boyle Journal of Cheminformatics volume 4, Article number: 22 (2012) Background: There are two line notations of chemical structures that have established themselves in the field: the SMILES string and the […]
Detection of IUPAC and IUPAC-like chemical names Roman Klinger, Corinna Kolá?ik, Juliane Fluck, Martin Hofmann-Apitius, Christoph M. Friedrich Bioinformatics, Volume 24, Issue 13, July 2008, Pages i268–i276, https://doi.org/10.1093/bioinformatics/btn181 Motivation: Chemical compounds like small signal molecules or other biological active chemical substances are an important entity class in life science publications and patents. Several representations and nomenclatures […]